OUR COMMITMENT
AUREPS
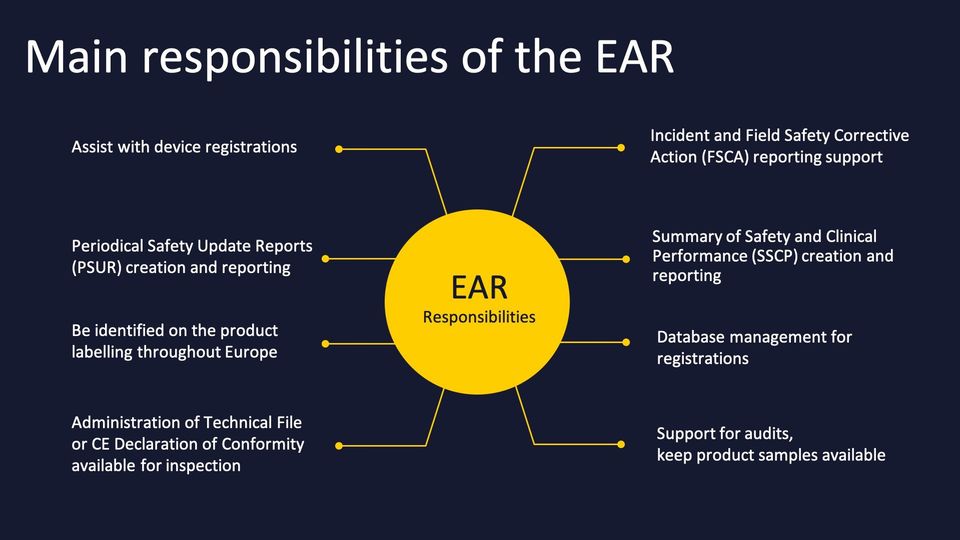
- serves as your Authorised European Representative (EAR)
- assures your compliance through conformity assessment procedures
- serves as primary contact to EU Authorities
- monitors your post-market surveillance including incident reporting

LEGAL REQUIREMENTS
MISSION
Our mission is to assist our clients in introducing innovative medical technologies to the European market and provide customized product commercialization and distribution models for new product development concepts. Our focus is on medical devices especially in the fields of prosthetic implants, surgery, dental technology, cardio-vascular surgery, neuromonitoring and aesthetic surgery.
PHILOSOPHY
It is this philosophy and dedication to service that has been the paradigm for AUREPS since its founding. AUREPS has successfully represented numerous companies in bringing important, cutting-edge technologies to the EU market.